Carotenoid Pigments Reflect Do Not Absorb Which Color of Light
8.2B: Absorption of Light
-
- Last updated
- Save as PDF
- Page ID
- 13207
Pigments, like chlorophyll and carotenoids, absorb and reflect light at a certain region of the electromagnetic spectrum.
Learning Objectives
- Differentiate between chlorophyll and carotenoids.
Key Points
- Plant pigment molecules absorb only light in the wavelength range of 700 nm to 400 nm; this range is referred to as photosynthetically-active radiation.
- Violet and blue have the shortest wavelengths and the most energy, whereas red has the longest wavelengths and carries the least amount of energy.
- Pigments reflect or transmit the wavelengths they cannot absorb, making them appear in the corresponding color.
- Chorophylls and carotenoids are the major pigments in plants; while there are dozens of carotenoids, there are only five important chorophylls: a, b, c, d, and bacteriochlorophyll.
- Chlorophyll a absorbs light in the blue-violet region, chlorophyll b absorbs red-blue light, and both a and b reflect green light (which is why chlorophyll appears green).
- Carotenoids absorb light in the blue-green and violet region and reflect the longer yellow, red, and orange wavelengths; these pigments also dispose excess energy out of the cell.
Key Terms
- chlorophyll: Any of a group of green pigments that are found in the chloroplasts of plants and in other photosynthetic organisms such as cyanobacteria.
- carotenoid: Any of a class of yellow to red plant pigments including the carotenes and xanthophylls.
- spectrophotometer: An instrument used to measure the intensity of electromagnetic radiation at different wavelengths.
Absorption of Light
Light energy initiates the process of photosynthesis when pigments absorb the light. Organic pigments have a narrow range of energy levels that they can absorb. Energy levels lower than those represented by red light are insufficient to raise an orbital electron to an excited, or quantum, state. Energy levels higher than those in blue light will physically tear the molecules apart, a process called bleaching. For example, retinal pigments can only "see" (absorb) 700 nm to 400 nm light; this is visible light. For the same reasons, plant pigment molecules absorb only light in the wavelength range of 700 nm to 400 nm; plant physiologists refer to this range for plants as photosynthetically-active radiation.
The visible light seen by humans as the color white light actually exists in a rainbow of colors in the electromagnetic spectrum, with violet and blue having shorter wavelengths and, thus, higher energy. At the other end of the spectrum, toward red, the wavelengths are longer and have lower energy.

Understanding Pigments
Different kinds of pigments exist, each of which has evolved to absorb only certain wavelengths or colors of visible light. Pigments reflect or transmit the wavelengths they cannot absorb, making them appear in the corresponding color.
Chlorophylls and carotenoids are the two major classes of photosynthetic pigments found in plants and algae; each class has multiple types of pigment molecules. There are five major chlorophylls: a, b, c and d, along with a related molecule found in prokaryotes called bacteriochlorophyll.
With dozens of different forms, carotenoids are a much larger group of pigments. The carotenoids found in fruit, such as the red of tomato (lycopene), the yellow of corn seeds (zeaxanthin), or the orange of an orange peel (β-carotene), are used to attract seed-dispersing organisms. In photosynthesis, carotenoids function as photosynthetic pigments that are very efficient molecules for the disposal of excess energy. When a leaf is exposed to full sun, the light-dependent reactions are required to process an enormous amount of energy; if that energy is not handled properly, it can do significant damage. Therefore, many carotenoids are stored in the thylakoid membrane to absorb excess energy and safely release that energy as heat.
Each type of pigment can be identified by the specific pattern of wavelengths it absorbs from visible light, which is the absorption spectrum. Chlorophyll a absorbs light in the blue-violet region, while chlorophyll b absorbs red-blue light. Neither a or b absorb green light; because green is reflected or transmitted, chlorophyll appears green. Carotenoids absorb light in the blue-green and violet region and reflect the longer yellow, red, and orange wavelengths.
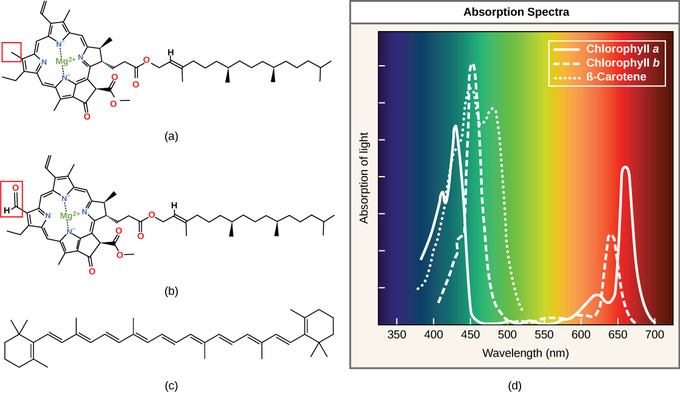
Many photosynthetic organisms have a mixture of pigments. In this way organisms can absorb energy from a wider range of wavelengths. Not all photosynthetic organisms have full access to sunlight. Some organisms grow underwater where light intensity and quality decrease and change with depth. Other organisms grow in competition for light. Plants on the rainforest floor must be able to absorb any light that comes through because the taller trees absorb most of the sunlight and scatter the remaining solar radiation

When studying a photosynthetic organism, scientists can determine the types of pigments present by using a spectrophotometer. These instruments can differentiate which wavelengths of light a substance can absorb. Spectrophotometers measure transmitted light and compute its absorption. By extracting pigments from leaves and placing these samples into a spectrophotometer, scientists can identify which wavelengths of light an organism can absorb.
Carotenoid Pigments Reflect Do Not Absorb Which Color of Light
Source: https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_General_Biology_(Boundless)/8%3A_Photosynthesis/8.2%3A_The_Light-Dependent_Reactions_of_Photosynthesis/8.2B%3A_Absorption_of_Light#:~:text=Neither%20a%20or%20b%20absorb,%2C%20red%2C%20and%20orange%20wavelengths.